Get Healthy!
- Posted September 25, 2023
Surgeons Perform Transplant of Gene-Tweaked Pig Heart Into Second Patient
A second human patient has received a genetically altered pig heart as he battles the ravages of end-stage heart disease.
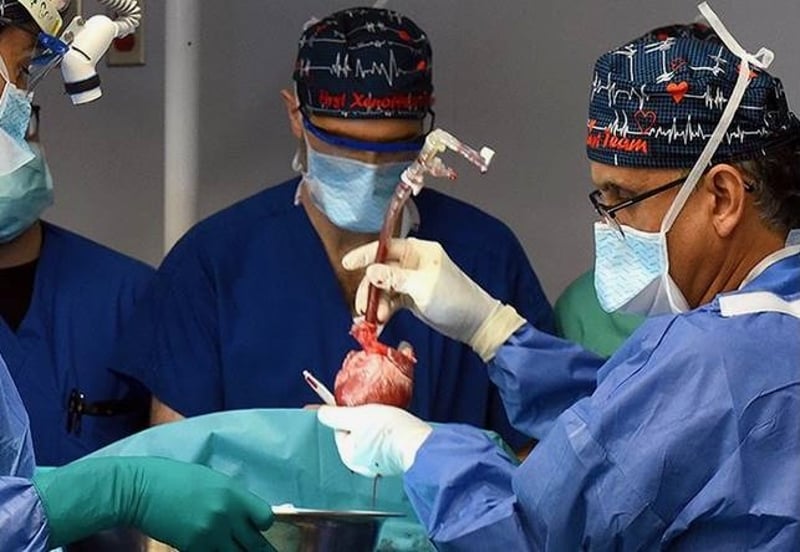
The 58-year-old man, Lawrence Faucette, received the pig organ at the University of Maryland Medical Center in Baltimore.
The medical team was the same one that performed the first pig transplant with another patient in January 2022.
"We are once again offering a dying patient a shot at a longer life, and we are incredibly grateful to Mr. Faucette for his bravery and willingness to help advance our knowledge of this field,"said Dr. Bartley Griffith, who transplanted the pig heart into both the first and second patients.
"We are hopeful that he will get home soon to enjoy more time with his wife and the rest of his loving family,"Griffith, a professor of transplant surgery and clinical director of the Cardiac Xenotransplantation Program, said in a medical center news release.
Faucette had been deemed ineligible for a transplant with a human heart because of preexisting peripheral vascular disease and complications with internal bleeding.
"My only real hope left is to go with the pig heart, the xenotransplant,"he said during an interview from his hospital room a few days before his surgery. "Dr. Griffith, Dr. Mohiuddin and their entire staff have been incredible, but nobody knows from this point forward. At least now I have hope, and I have a chance."
"We have no expectations other than hoping for more time together,"added his wife, Ann Faucette. "That could be as simple as sitting on the front porch and having coffee together."
A 58-year-old father of two from Frederick, Md., Faucette is currently breathing on his own. His heart is functioning well without supportive devices. A 20-year Navy veteran, he was a lab technician at the National Institutes of Health before retiring.
Without the transplant, he would almost certainly have died from heart failure.
The xenotransplant was approved on Sept. 15 by the U.S. Food and Drug Administration under its single patient investigational new drug (IND) "compassionate use"pathway, which allows for an experimental product to be used for someone with a life-threatening medical condition.
Faucette's case could provide important groundwork in future lifesaving surgery.
"We are continuing to pursue the pathway to clinical trials by providing important new data on pre-clinical research that has been requested by the FDA,"said Dr. Muhammad Mohiuddin, a professor of surgery at Maryland.
Mohiuddin joined the faculty seven years ago and established the Cardiac Xenotransplantation Program. He co-led this latest procedure with Griffith.
"The FDA used our data from these new studies, as well as our experience with the first patient, to determine that we were ready to attempt a second transplant in an end-stage heart disease patient who had no other treatment options,"Mohiuddin said.
The pig heart was provided by United Therapeutics Corp., through its xenotransplantation subsidiary Revivicor, based in Blacksburg, Va. The company has funded a $22 million research program to test pig hearts from Revivicor in baboons.
The surgical team removed the pig heart on the day of the surgery and put it in what's called the XVIVO Heart Box, which preserved it.
Ten gene edits were made in the donor pig. Those involved knocking out three genes in the pig that could be involved in rejection of pig organs in the human body and one that will help prevent excessive growth of pig heart tissue. In addition, six human genes responsible for immune acceptance of the pig heart were inserted into the genome of the pig.
"This procedure is another significant step forward in bringing our vision of lifesaving xenotransplantation to those patients in desperate need,"David Ayares, president and chief scientific officer of Revivicor, said in a company news release.
Faucette is also being treated with conventional anti-rejection drugs and a novel antibody therapy. The latter is experimental. Called tegoprubart, it blocks CD154, a protein involved in immune system activation.
He is among about 110,000 Americans currently waiting for an organ transplant. More than 6,000 people die each year on the transplant list, according to organdonor.gov.
Among the fears about transplanting animal organs to humans are concerns about spreading diseases between animals and humans. Another is triggering a dangerous immune response.
Faucette underwent psychiatric evaluation and consented to the surgery's many risks.
The first patient to receive a pig heart, David Bennett, later died from heart failure. His death was likely due in part to poor health prior to the surgery.
After that surgery, the pig heart functioned well for several weeks with no signs of acute rejection.
The surgical team spent five years perfecting the surgical technique on non-human primates before the two human transplants.
More information
The U.S. Food and Drug Administration has more on xenotransplantation.
SOURCE: University of Maryland School of Medicine, news release, Sept. 22, 2023
